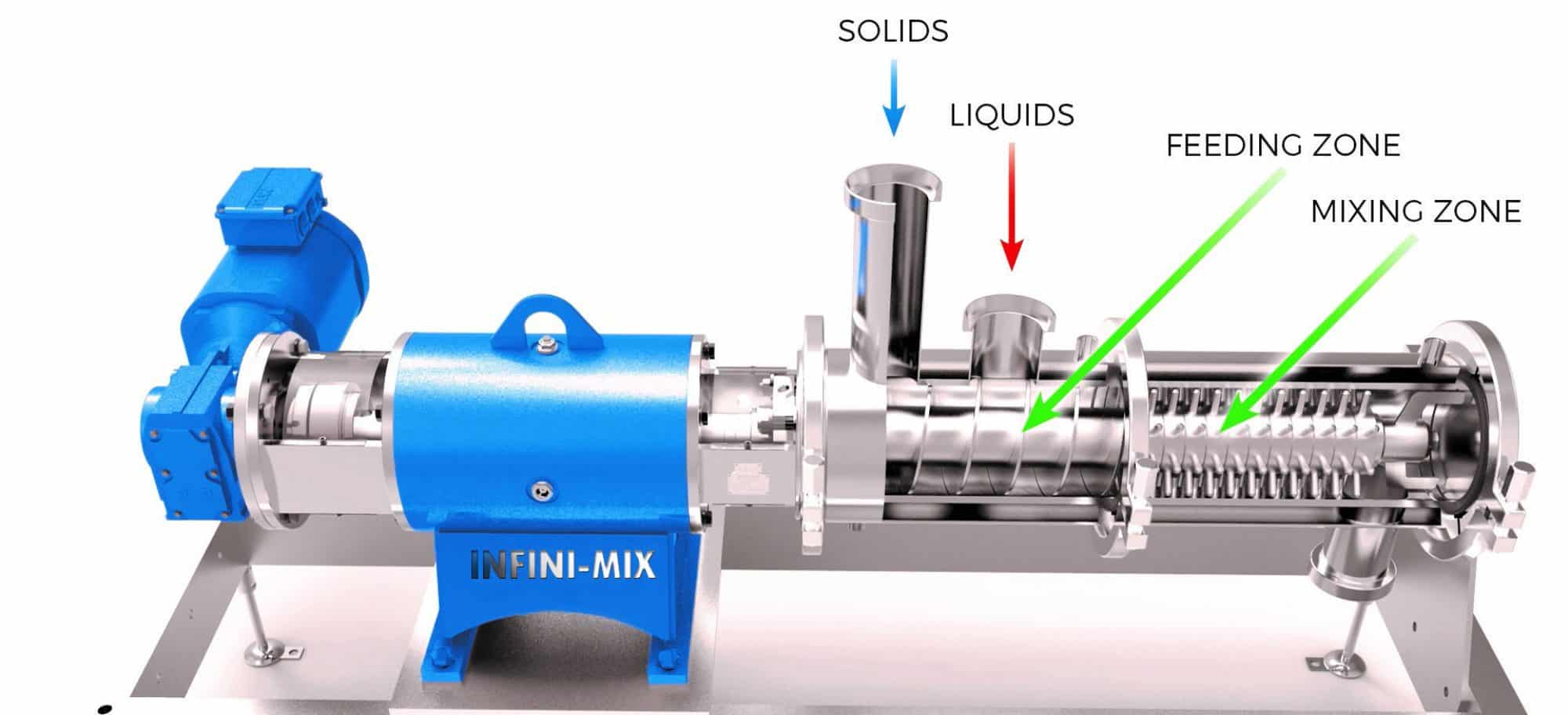
Mixing Terminology
All too often, manufacturers and suppliers alike, uses terms incorrectly. In the manufacturing world, there can be no misunderstandings. Do you know the differences between a “continuous process” and a “continuous batch process?” We decided to put together this “Mixing Terminology” section to help our customers (and some of our competitors) get on the same page when selecting the proper mixing solution.
If you have any ideas of additional terms you would like to see, click HERE to email our experts.
Batch Mixing
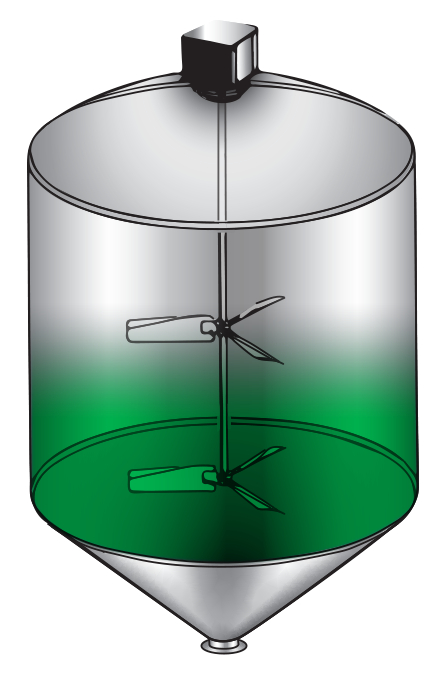
All ingredients are metered into a single tank and blended. Once the blend is complete, the final product is sent to a pasteurizer or to packaging. No product is being packaged while a batch is being made. The limitation of the run is based on the size of the batch tank. Recipe changes require a cleaning of the batch vessel and all of the piping and equipment associated with the recipe. Batch mixing is typically labor intensive and produces inconsistent product.
Continuous Batch Mixing
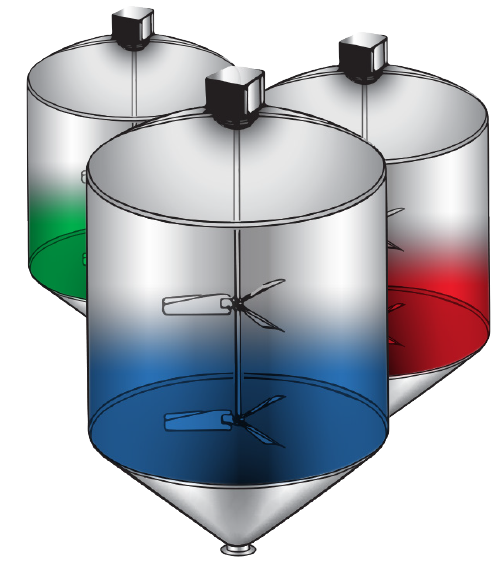
Often confused with Continuous Mixing, Continuous Batch Mixing is where all ingredients are metered into multiple tanks and blended. As one tank is being processed forward to packaging, the next batch is being made in one of the other tanks. Product is continually being packaged and the limitation of the run is based on the amount of ingredients on hand. Recipe changes require a cleaning of the batch vessel and all of the piping and equipment associated with the recipe. Continuous Batch Mixing is labor intensive and produces inconsistent product.
Recirculated Batch Mixing
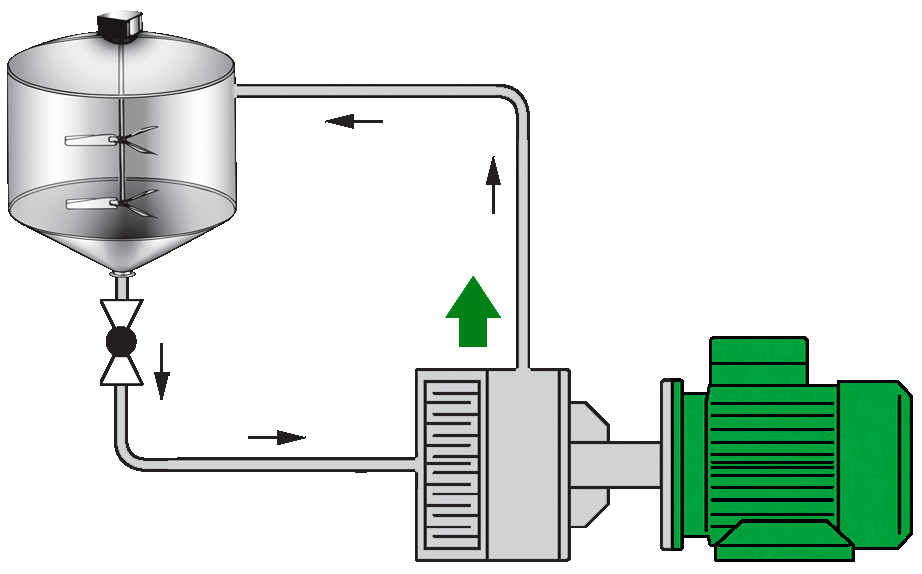
All ingredients are metered into a single tank. A pump on the discharge of the vessel recirculates the product through an inline mixer and returns back to the vessel. The product is recirculated until it is deemed homogenous. One inline mixer can service multiple tanks but can only mix one tank at a time.
Continuous Process
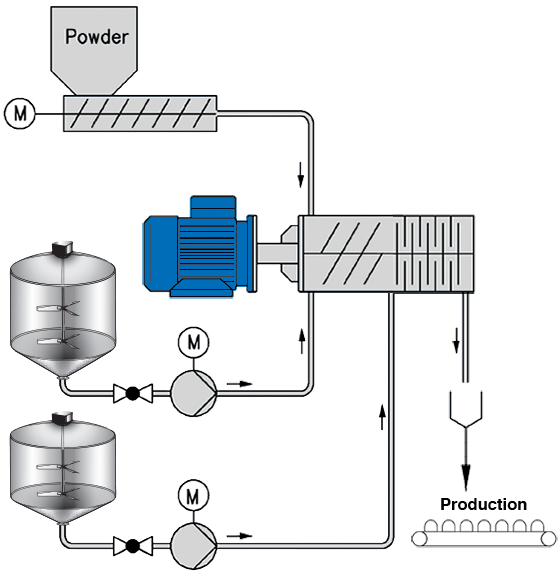
All ingredients are metered into a common line or an inline mixer. The product seamlessly goes through a single pass system to packaging. The limitation of the run is based on the amount of ingredients you have on hand. Recipe changes require a cleaning of the all of the piping and equipment associated with the recipe.
Late Stage Differentiation
Late Stage Differentiation – or “End of Pipe” applications are where a final ingredient (or set of ingredients), can be added to the primary liquid at the end of the process or at the filler. These final ingredients can be delicate inclusions, fragrances, coloring or virtually anything.
Single Pass System vs. Recirculating
A single pass system means that the mixture will only be able to pass through the inline mixer one time before forward flow. A single pass system can be a batch, continuous batch or continuous system. In a recirculating system, the mixture makes several (2 or more) passes through the inline mixer. For homogeneity, the number of times the mixture must pass through the mixer is a function of the type of ingredients and the intensity of the mixing.
Mixture
A “mixture” contains two or more substances that are not chemically combined. The substances in a mixture can be separated using physical methods such as filtration, freezing, and distillation because, when mixed, individual substances keep their properties in a mixture, while if they form a compound their properties can change.
Emulsion
An “emulsion” is a fine dispersion of minute droplets of one liquid in another in which it is not soluble or miscible. Oil and water is a common example of an emulsion. Though a high shear mixer can blend water and oil together to make it look homogeneous, the liquids will eventually separate out over time. The amount of time it will take to separate is based on the level of mixing intensity.
Suspension
A “suspension” is a heterogeneous mixture in which solid particles eventually settle out of a solution-like phase sometime after their introduction. An example of a “suspension” would be the coatings that are sprayed onto Doritos or other chips. These are typically a powdered flavoring dispersed in an oil.
Solution
A “solution” is a liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent). A good example is sugar and water or salt and water. The sugar or salt solids will ultimately dissolve into the water over time. Heat and shear can decrease the amount of time required to dissolve.
Miscibile vs. Immiscible
Two substances are said to be “miscible” if they mix in all proportions to form a homogeneous solution. They must fully dissolve in each other at any concentration. Substances that are “immiscible” if there are certain proportions in which the mixture does not form a solution. These terms can be used to describe liquids, solids and gases.
Tip Speed
TIP SPEED = ROTOR DIAMETER x 3.1415 x RPM
Tip speed is required for calculating the shear forces being exerted on a given product.
Shear Rate
SHEAR RATE = TIP SPEED / GAP
Shear rate is the rate at which fluid layers move past each other.
Viscosity
The viscosity of a fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress. The shear resistance in a fluid is caused by inter-molecular friction exerted when layers of fluid attempt to slide by one another.1
(see our viscosity reference sample videos)